


Physiological-mimicking dissolution tester for transfer model prediction.
Kanadevia’s GIS system, validated by numerous peer-reviewed studies
—including those from the University of Michigan—offers enhanced functionality and scalability.
Recognized for its performance for Class IIb formulations, GIS-α is an advanced in vivo predictive dissolution testing system.
It uniquely simulates the entire GI tract using a sophisticated transfer model for prediction of drug absorption profiles.

While conventional dissolution testers are effective for quality control purposes, they fall short in mimicking in vivo drug behavior or accurately predicting bioequivalence (BE) study outcomes.
With growing demand for full GI tract evaluation, USP I and II remain unable to mimic stomach-to-intestine physiology.
Both dissolution and absorption is crucial in formulation testing, yet current dissolution apparatuses fall short.
As Class II and IV drugs become more prevalent, pH shifts between the stomach and intestines can lead to precipitation or supersaturation, often complicating the prediction of in vivo behavior.
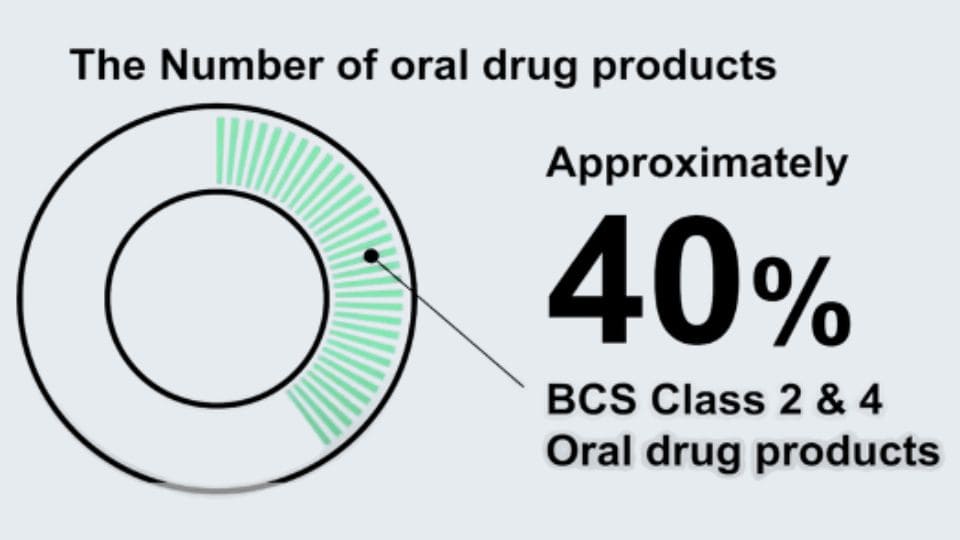
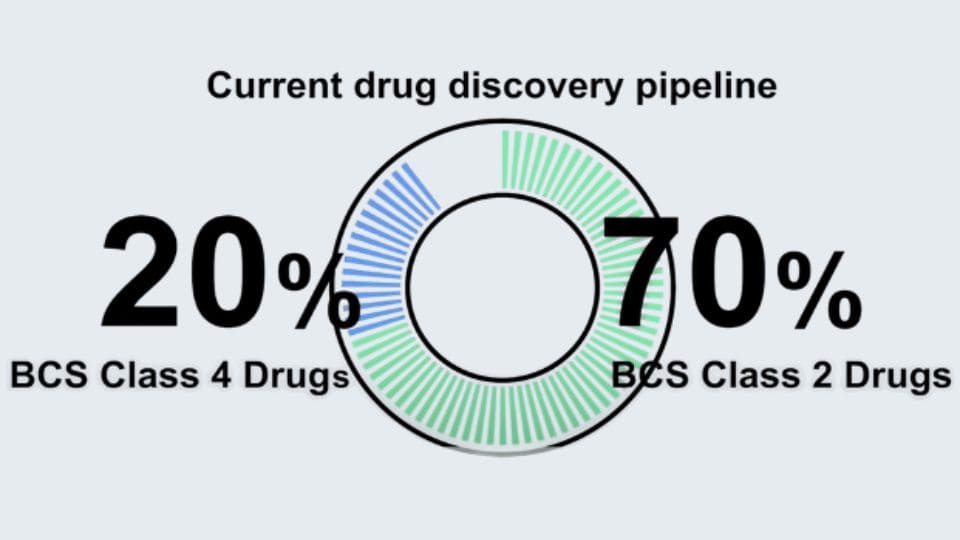
From in vitro to in vivo — a physiologically inspired transfer model.
PScientifically validated by the PQRI consortium and numerous studies, this dissolution tester is designed to simulate physiological conditions in a functionally relevant manner.
The formulation is sequentially transferred from the stomach to the duodenum, jejunum, and ileum.
To simulate physiological pH conditions, simulated gastric fluid is used for the stomach, and phosphate buffer etc, is applied in the duodenum.
A biphasic model with an aqueous (dissolution) layer and an oil (absorption) layer representing the jejunum or ileum has been reported to enable observation of absorption behavior.
Functional, user-friendly, and made in Japan.s

For inquiries about products and recruitment, please contact us here.
Contact Us